Aesthetic procedures are really popular nowadays, people seek their personal fulfillment through some methods, in this content we will talk about how to register cannula for aesthetic procedure.
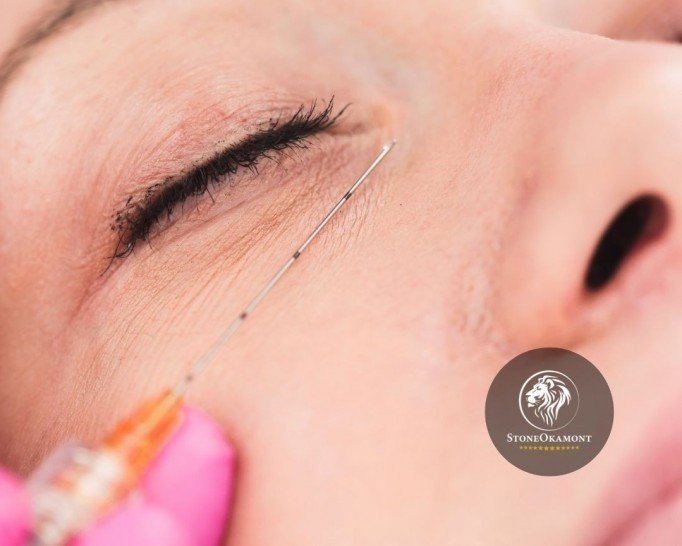
The cannula for aesthetic procedure is widely used on the skin because it causes less bruising, marks or swelling.
What is the cannula for aesthetic procedure?
The Cannula is a small tube, made of different materials, which can be plastic or metal, varying in size, shape and objectives, intended to be introduced into the body.
Specifically speaking about the cannula for cosmetic procedures, it is significantly longer compared to a needle and more flexible, allowing a smoother and more continuous distribution, for example in a lip filler. It can also be considered a great option for those who are afraid of needles.
Do you intend to register cannula for aesthetic procedure? Count on Stone Okamont, request your quote.
What is needed to register cannula for aesthetic procedure?
In order to register the cannula for an aesthetic procedure, it is necessary to go through some regulatory steps, these steps depend on each other to finally obtain the cannula registration, which fits into the last step of the whole process.
The first step is the regularization of your company, obtaining the Operating License, this is done at VISA – Local Sanitary Surveillance. VISA will initially carry out an inspection of your establishment pointing out several issues that vary from sanitary matters, plant of the establishment and others.
The second step to obtain registration of the cannula for the aesthetic procedure is the Company Operating Authorization, this part of the process takes place in Brasilia with ANVISA - National Agency for Sanitary Surveillance, at this stage a compilation of documents is made that must be attached to the report received at the end of the Operating License process.
What is the risk classification of the cannula for aesthetic procedure?
Before registering the cannula for aesthetic procedures, it is first necessary to classify it.
Anvisa classifies cannula for aesthetic procedures as a correlate of risk class II – Medium Risk. As the product is classified as medium risk, the Certificate of Good Manufacturing Practices - CBPF is not required, this is mandatory for companies with products classified as risky class III - High risk. The Good Manufacturing Practices consist of a set of norms and rules that cover all the processes that involve the product.
Completed steps! You can now register cannula for aesthetic procedure.
After completing all these steps, it is finally possible to register a cannula for an aesthetic procedure. In this phase, information about the product is presented, such as: efficacy and safety tests, material, use instructions and others.
Sounds complicated? Do not worry! Stone Okamont is here to help you! With trained professionals in several areas, we provide all the necessary advice so that your company and product regularization process is fast, practical and hassle-free! In this way offering the best cost benefit for your business!
Contact us! Fill out the form below, talk to one of our professionals and have all your doubts answered about how to register a cannula for aesthetic procedures.