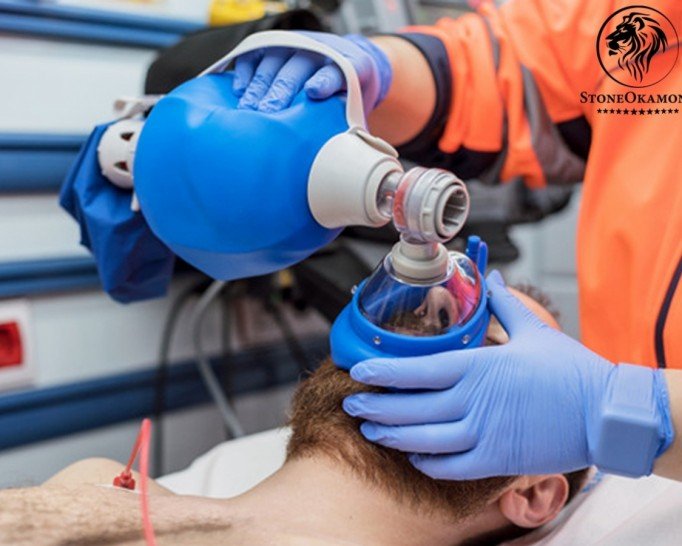
The AMBU is a manual device to perform cardiopulmonary resuscitation. The equipment works by taking compressed air into the lungs in case of respiratory arrest, heart attack, drowning and others. The AMBU assists in pulmonary ventilation until the patient can be connected to automatic artificial ventilation machines.
The equipment is generally used in first aid where immediate care is required. The AMBU is a dismountable and sterilizable equipment.
Anvisa classifies the AMBU as a correlate risk class II - medium risk. It is not necessary to obtain the Certificate of Good Manufacturing Practices (CBPF) to register Artificial Manual Breathing Unit in Brazil. CBPF is mandatory for companies that work with drugs and products with risk class III - High risk and IV - Maximum risk. It is a set of rules and procedures to establish a standard in relation to the stages of production until delivery to the final consumer. Learn more: Certificate of Good Manufacturing Practices
Request a budget
Anvisa (National Health Surveillance Agency) is the Brazilian agency responsible for regulating products: cosmetics, medicines, food, correlate products, sanitizing agents and others.
Do you know what it takes to register Artificial Manual Breathing Unit in Brazil? In this content we will briefly discuss each regulatory step necessary to register Artificial Manual Breathing Unit in Brazil.
What do you need to register Artificial Manual Breathing Unit in Brazil?
To register Artificial Manual Breathing Unit in Brazil, it is important that the company is properly regularized. This regularization can be carried out in two ways. (See below for the company regularization alternatives).
Once the company is regularized, in order to register Artificial Manual Breathing Unit in Brazil, it will be necessary to submit tests to Anvisa that prove the safety and efficacy of the equipment, in order to ensure that it will perform its function within the expected period and without causing any type of damage to the patient. In addition, specific information about the product will be requested, such as: Composition material, form of sterilization, instruction manual and others. Learn more: Product registration.
Company Regularization (Option 1)
To register Artificial Manual Breathing Unit in Brazil, the company needs to be regularized with Anvisa. For this, it is necessary that the company establishes a physical location in Brazil following all the requirements imposed by Visa and Anvisa.
After the establishment of the physical location, the Operating License process begins. This is carried out with the local or state Visa (Local Health Surveillance). At this stage, the company must adapt its structure in accordance with the RDC (Resolution of the Collegiate Board) related to the activity performed in order to receive an inspection from a Visa inspection agent.
Operating permits is the last company regularization process for register Artificial Manual Breathing Unit in Brazil. This process is now federal in scope, being protocoled in Brasília, and verified by Anvisa. At this point in the process, petitions are verified, documents are compiled, fees are collected and others.
Holder services (Option2)
Stone Okamont also offers the Holder Service option. With this option, your products can be marketed in Brazil without the need to establish a physical location. This process is done through the BRB Holder, requiring only the registration of products.
Talk to those who understand!
Register Artificial Manual Breathing Unit in Brazil can be a very complicated task without a competent advice to assist in all processes. Stone Okamont has trained professionals in several areas of expertise to better serve you! Count on us to register Artificial Manual Breathing Unit in Brazil!
Fill out the form below, talk to one of our professionals and ask all your questions about how to register Artificial Manual Breathing Unit in Brazil.